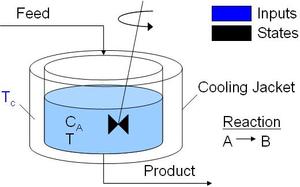
Stirred Reactor
Apps.StirredReactor History
Show minor edits - Show changes to markup
</pre></font> (:htmlend:)
Van de Vusse Reactor
The Van de Vusse reaction kinetics are employed in many benchmarking problems. This model is a simple stirred tank reactor model with reactions A->B->C and A->2D. Note that in the original reference, the reactor volume was listed as 0.01 L. This model has been modified for the original intent of 10 L reactor volume.
(:html:)<font size=2><pre>
Continuously Stirred Tank Reactor with energy
balance and reactions A->B->C and A->2D
Model cstr
Parameters
F = 14.19 ! Feed rate (l/hr)
Qk = -1579.5 ! Jacket cooling rate (kJ/hr)
Ca0 = 5.1 ! Inlet feed concentration (mol/m^3)
T0 = 104.9 ! Inlet feed temperature (degC)
k10 = 1.287e12 ! A->B Pre-exponential factor (1/hr)
k20 = 1.287e12 ! B->C Pre-exponential factor (1/hr)
k30 = 9.043e9 ! A->2D Pre-exponential factor (1/hr)
E1 = 9758.3 ! A->B Activation Energy (K)
E2 = 9758.3 ! B->C Activation Energy (K)
E3 = 8560 ! A->2D Activation Energy (K)
dHrAB = 4.2 ! A->B Heat of Reaction (kJ/mol A)
dHrBC = -11 ! B->C Activation Energy (kJ/mol B)
dHrAD = -41.85 ! A->2D Activation Energy (kJ/mol A)
rho = 0.9342 ! density (kg/l)
Cp = 3.01 ! Heat capacity of reactants (kJ/kg-K)
kw = 4032 ! Heat transfer coefficient (kJ/h-K-m^2)
AR = .215 ! Area of jacket cooling (m^2)
VR = 10.0 ! Reactor volume (l)
mK = 5 ! Mass of cooling (kg)
CpK = 2 ! Heat capacity of cooling (kJ/kg-K)
End Parameters
Variables
! Differential States
Ca = 2.2291 ! Concentration of A in CSTR (mol/l)
Cb = 1.0417 ! Concentration of B in CSTR (mol/l)
Cc = 0.91397 ! Concentration of C in CSTR (mol/l)
Cd = 0.91520 ! Concentration of D in CSTR (mol/l)
T = 79.591 ! Temperature in CSTR (degC)
Tk = 77.69 ! Cooling jacket temperature (degC)
End Variables
Intermediates
k1 = k10*exp(-E1/(T+273.15))
k2 = k20*exp(-E2/(T+273.15))
k3 = k30*exp(-E3/(T+273.15))
End Intermediates
Equations
! note: the $ denotes time differential
! (e.g. $x is dx/dt)
! species balances
VR * $Ca = -k1*VR*Ca - k3*VR*Ca^2 + F*(Ca0-Ca)
VR * $Cb = k1*VR*Ca - k2*VR*Cb - F*Cb
VR * $Cc = k2*VR*Cb - F*Cc
VR * $Cd = k3*VR*Ca^2 - F*Cd
! energy balance on reactor
rho*Cp*VR*$T = F*rho*Cp*(T0 - T) &
- VR*(k1*Ca*dHrAB + k2*Cb*dHrBC + k3*Ca^2*dHrAD) &
+ kw*AR*(Tk - T)
! energy balance on cooling
mK * CpK * $Tk = Qk + kw*AR*(T - Tk)
End Equations
End Model
The Van de Veer reaction kinetics are employed in many benchmarking problems. This model is a simple stirred tank reactor model with reactions A->B->C and A->2D. Note that in the original reference, the reactor volume was listed as 0.01 L. This model has been modified for the original intent of 10 L reactor volume.
The Van de Vusse reaction kinetics are employed in many benchmarking problems. This model is a simple stirred tank reactor model with reactions A->B->C and A->2D. Note that in the original reference, the reactor volume was listed as 0.01 L. This model has been modified for the original intent of 10 L reactor volume.
Van de Veer Reactor
The Van de Veer reaction kinetics are employed in many benchmarking problems. This model is a simple stirred tank reactor model with reactions A->B->C and A->2D. Note that in the original reference, the reactor volume was listed as 0.01 L. This model has been modified for the original intent of 10 L reactor volume.
(:htmlend:)
(:htmlend:)
The Van de Veer reaction kinetics are employed in many benchmarking problems. This model is a simple stirred tank reactor model with reactions A->B->C and A->2D. Note that in the original reference, the reactor volume was listed as 0.01 L. This model has been modified for the original intent of 10 L reactor volume.
(:html:)<font size=2><pre>
Continuously Stirred Tank Reactor with energy
balance and reactions A->B->C and A->2D
Model cstr
Parameters
F = 14.19 ! Feed rate (l/hr)
Qk = -1579.5 ! Jacket cooling rate (kJ/hr)
Ca0 = 5.1 ! Inlet feed concentration (mol/m^3)
T0 = 104.9 ! Inlet feed temperature (degC)
k10 = 1.287e12 ! A->B Pre-exponential factor (1/hr)
k20 = 1.287e12 ! B->C Pre-exponential factor (1/hr)
k30 = 9.043e9 ! A->2D Pre-exponential factor (1/hr)
E1 = 9758.3 ! A->B Activation Energy (K)
E2 = 9758.3 ! B->C Activation Energy (K)
E3 = 8560 ! A->2D Activation Energy (K)
dHrAB = 4.2 ! A->B Heat of Reaction (kJ/mol A)
dHrBC = -11 ! B->C Activation Energy (kJ/mol B)
dHrAD = -41.85 ! A->2D Activation Energy (kJ/mol A)
rho = 0.9342 ! density (kg/l)
Cp = 3.01 ! Heat capacity of reactants (kJ/kg-K)
kw = 4032 ! Heat transfer coefficient (kJ/h-K-m^2)
AR = .215 ! Area of jacket cooling (m^2)
VR = 10.0 ! Reactor volume (l)
mK = 5 ! Mass of cooling (kg)
CpK = 2 ! Heat capacity of cooling (kJ/kg-K)
End Parameters
Variables
! Differential States
Ca = 2.2291 ! Concentration of A in CSTR (mol/l)
Cb = 1.0417 ! Concentration of B in CSTR (mol/l)
Cc = 0.91397 ! Concentration of C in CSTR (mol/l)
Cd = 0.91520 ! Concentration of D in CSTR (mol/l)
T = 79.591 ! Temperature in CSTR (degC)
Tk = 77.69 ! Cooling jacket temperature (degC)
End Variables
Intermediates
k1 = k10*exp(-E1/(T+273.15))
k2 = k20*exp(-E2/(T+273.15))
k3 = k30*exp(-E3/(T+273.15))
End Intermediates
Equations
! note: the $ denotes time differential
! (e.g. $x is dx/dt)
! species balances
VR * $Ca = -k1*VR*Ca - k3*VR*Ca^2 + F*(Ca0-Ca)
VR * $Cb = k1*VR*Ca - k2*VR*Cb - F*Cb
VR * $Cc = k2*VR*Cb - F*Cc
VR * $Cd = k3*VR*Ca^2 - F*Cd
! energy balance on reactor
rho*Cp*VR*$T = F*rho*Cp*(T0 - T) &
- VR*(k1*Ca*dHrAB + k2*Cb*dHrBC + k3*Ca^2*dHrAD) &
+ kw*AR*(Tk - T)
! energy balance on cooling
mK * CpK * $Tk = Qk + kw*AR*(T - Tk)
End Equations
End Model </pre></font> (:htmlend:)
The Van de Veer reaction kinetics are employed in many benchmarking problems. This model is a simple stirred tank reactor model with reactions A->B->C and A->2D. Note that in the original reference, the reactor volume was listed as 0.01 L. This model has been modified for the original intent of 10 L reactor volume.
(:html:)<font size=1><pre>
(:html:)<font size=2><pre>

(:html:)<font size=1><pre>
APMonitor Modeling Language
https://www.apmonitor.com
CSTR model from
Michael A. Henson and Dale E. Seborg. Nonlinear
Process Control. Prentice Hall PTR, Upper
Saddle River, New Jersey, 1997.
Description:
Continuously Stirred Tank Reactor with energy
balance and reaction A->B.
The temperature of the cooling
jacket is the control.
Model cstr
Parameters
! Manipulated Variables
Tc = 270 ! Temperature of cooling jacket (K)
! Parameters
q = 100 ! Volumetric Flowrate (m^3/sec)
V = 100 ! Volume of CSTR (m^3)
rho = 1000 ! Density of A-B Mixture (kg/m^3)
Cp = .239 ! Heat capacity of A-B Mixture (J/kg-K)
mdelH = 5e4 ! Heat of reaction for A->B (J/mol)
! E - Activation energy in the
! Arrhenius Equation (J/mol)
! R - Universal Gas Constant
! = 8.31451 J/mol-K
! EoverR = E/R
EoverR = 8750
k0 = 7.2e10 ! Pre-exponential factor (1/sec)
! U - Overall Heat Transfer
! Coefficient (W/m^2-K)
! A - Area - this value is specific
! for the U calculation (m^2)
! UA = U * A
UA = 5e4
Caf = 1 ! Feed Concentration (mol/m^3)
Tf = 350 ! Feed Temperature (K)
End Parameters
Variables
! Differential States
Ca = 0.9 ! Concentration of A in CSTR (mol/m^3)
T = 305 ! Temperature in CSTR (K)
End Variables
Equations
! note: the $ denotes time differential
! (e.g. $x is dx/dt)
! mole balance for species A
V * $Ca = q*(Caf-Ca) - k0*V*exp(-EoverR/T)*Ca
! energy balance
rho*Cp*V * $T = q*rho*Cp*(Tf - T) + V*mdelH*k0*exp(-EoverR/T)*Ca + UA*(Tc-T)
End Equations
End Model
</pre></font> (:htmlend:)
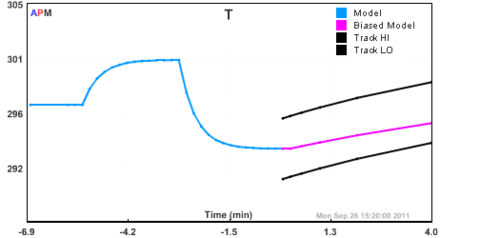
Reactor
Continuously Stirred Tank Reactor
The continuously stirred tank reactor is a popular model for benchmarking. It is a simple A to B reaction and has exothermic reaction instability with a prolonged cooling jacket temperature above 305 K.